
30
DECEMBER 2014
•
WORLD AQUACULTURE
•
WWW.WA S.ORGincreasing production mirrors
regional skills in capturing glass
eels and transporting them with
minimum stress to aquaculture
farms and in stocking and feeding
for maximum yield, which has
advanced greatly.
A government policy to
protect freshwater resources in
1997 directed the majority of
inland farms to terminate eel
aquaculture and subsequently
production moved to indoor
systems. A total of 236 farms
covering about 133 ha comprising
202 flow-through systems and 34
recirculating system farms in the Jeon-nam area were exclusively
designed and adopted for eel aquaculture (KSNO 2009). Indoor
systems for eel farming have always been under scrutiny for
improvement of quality, with the view to increase the overall
efficiency of farms. Flow-through systems with concrete bottoms
have been replaced by polypropylene polymer-bottom circular
tanks. Circular tanks ranging from 30-50 m
2
up to 200-300 m
2
are
used, based on eel life-stage.
The Need to Establish Nutrient Requirements
There are a number of constraints that need to be resolved
to develop a complete technology package for eel aquaculture.
Poor understanding of nutrient requirements and the availability
of balanced diets are major barriers to further expansion of eel
aquaculture. The greatest mortality at eel farms has been reported
during the weaning period and the adaptation period to dry feed.
Inasmuch as it is unlikely capture production will increase fur-
ther, studies have been conducted to boost aquaculture production
to meet the ever-increasing demand for this species (Lee and Bai
1997, Okorie
et al
. 2007, Bae
et al
. 2008). Our research center (FF-
NRC) has been conducting a series of experiments with different
age groups of Japanese eel to reevaluate the nutrient requirements,
the efficiency of various dietary ingredients and additives and the
body composition of wild and cultured eel. Here we present some
important micronutrient requirements, namely those for vitamin C,
vitamin E and arachidonic acid, in Japanese eel.
Vitamin C Requirement
Most marine and freshwater teleosts are unable to synthesize
vitamin C (ascorbic acid / AA) from D glucose because of the lack
of an enzyme, L-gulonolactone oxidase, that is responsible for the
synthesis of vitamin C
de novo
(Dabrowski 1990, Fracalossi
et
al
. 2001, Wilson 1973). In general, marine and freshwater teleosts
depend fully on a dietary supply of ascorbic acid.
Many general physiological functions of L-ascorbic acid
(AA) are well defined, the most important among them being
its capacity to act as a co-factor in the hydroxylation of proline
to hydroxyproline, critical for the helical structure of collagen.
L-ascorbic acid is also the most powerful reducing agent available
to cells, losing two hydrogen atoms to become dehydroascorbic
acid, and is of general importance as an antioxidant because of
its high reducing potential (Bai
2001).
The standard reference for
aquatic species nutrition,
Nutrient
Requirements of Fish and Shrimp
(NRC 2011), has documented the
studies devoted to evaluating AA
requirements in economically
important species. The previous
edition (NRC 1993) listed only
a few economically important
species in the vitamin C section,
but the current edition covers
the majority of commercially
important species.
A number of symptoms
linked to vitamin C deficiency, such as impaired collagen
formation, spinal deformation, haemorrhaging, retarded growth
and depressed immunity (Ai
et al.
2006, Al-Amoudi
et al
. 1992,
Gouillou-Coustans
et al
. 1998, Halver
et al.
1969) were formerly
common problems encountered at aquaculture farms. Knowledge
of vitamin C requirements has advanced substantially. As a result,
fish producers have been relieved of severe economic losses linked
to high and frequent incidence of malformed fish and subsequent
mortality.
Large discrepancies in quantitative requirements of vitamin C
in and between fish species are a result of differences in species, size
and methodological approaches and experimental conditions (NRC
1993). The dietary source of AA used in different experiments
is another major and fundamental difference, which makes it
complex to oversimplify the quantitative requirement of vitamin
C. L-ascorbic acid is the traditionally used vitamin C source in fish
and shrimp feeds, but it is thermolabile, unstable and easily oxidized
to an inactive form during feed processing and storage. Various
derivatives of AA, including L-ascorbyl-2-sulfate (C2S), L-ascorbyl-
2-monophosphate-Mg (C2MP-Mg), L-ascorbyl-2-monophosphate-
Ca (C2MP-Ca), L-ascorbyl-2-polyphosphate (C2PP) and ascorbate-
2-glucose (C2D), are more stable than the parent compound and
provide antiscorbutic activity in fish and shrimp.
The dietary vitamin C requirement of Japanese eel has been
estimated using L-ascorbic acid Ca as the source of vitamin C
(Ren
et al
. 2005). In an experiment to reevaluate the vitamin C
requirement in juvenile eel using L-ascorbyl-2-monophosphate
(AMP) as the vitamin C source, survival of eels fed the AMP-
supplemented diets was significantly greater than those of fish that
did not receive vitamin C supplementation. No vitamin C could be
detected in the whole body of fish fed AMP0 diet. The vitamin C
level in fish fed AMP108 diet was significantly greater than that of
eels fed AMP24 and AMP52. The vitamin C level of fish fed the
AMP1137 diet was significantly greater than those in fish fed all
other diets.
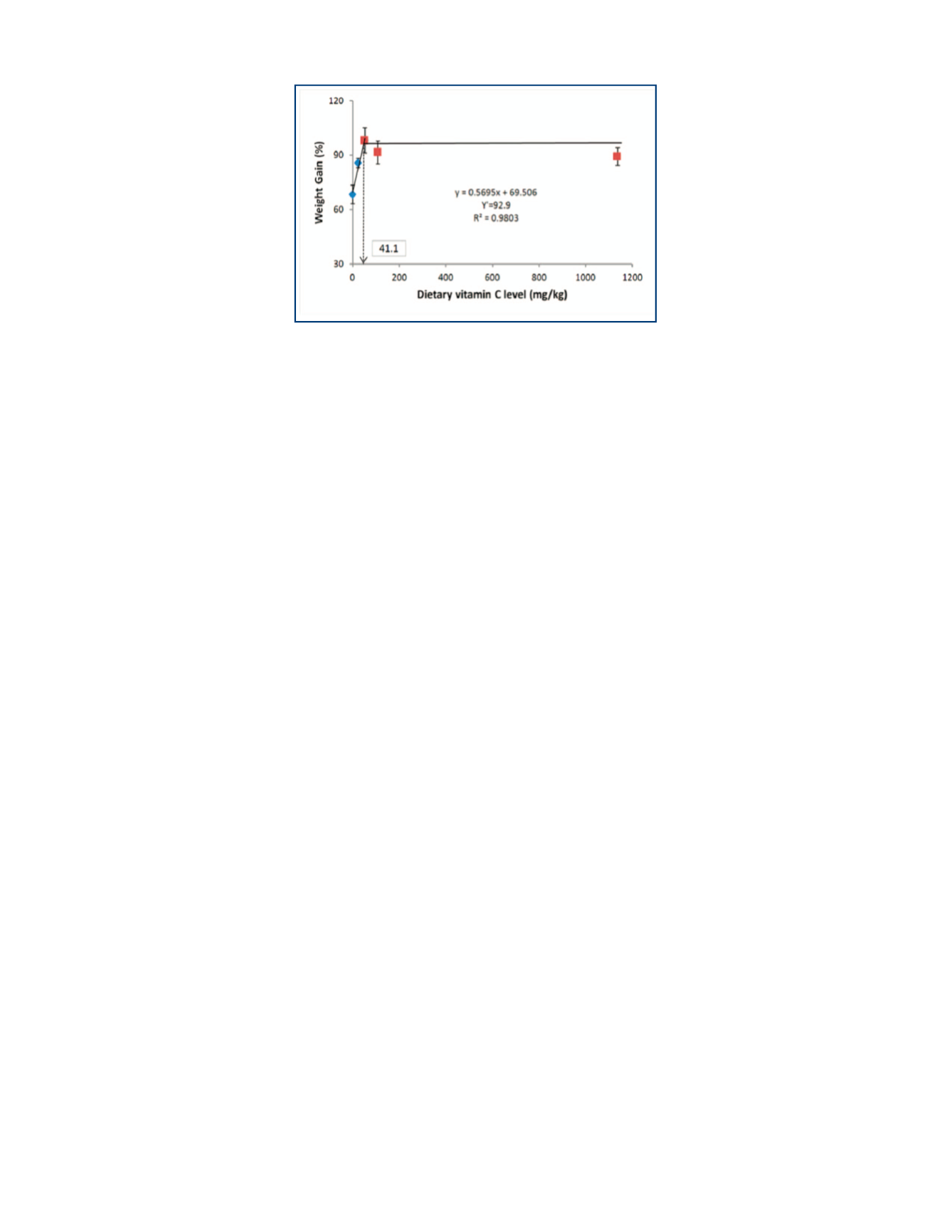
Dietary vitamin C in juvenile eel is essential. However, there
seems to be no benefit of increasing vitamin C supplementation in
diets beyond 24 mg AMP/kg diet, inasmuch as fish fed any of the
vitamin C supplemented diets had similar growth performance. The
requirement based on broken-line analysis of weight gain (Fig. 3)
is comparable to values obtained in common carp
Cyprinus carpio
TOP, FIGURE 3.
Broken-line analysis of vitamin C requirement of
Japanese eel based on weight gain.
















