
 WWW.WA
S.ORG
WWW.WA
S.ORG
•
WORLD AQUACULTURE
•
DECEMBER 2014
31
(45 mg AA/kg diet) (Gouillo-
Coustan
et al
. 1998). Ren
et al
.
(2005) reported the optimum
dietary level of AA for Japanese
eel juvenile growth to be more
than 27 mg AA/kg diet without
stating an upper limit. Although
no significant differences were
recorded above this minimum
level, specific growth rate
continued to increase up to the
maximum supplementation level.
Any differences in the minimum
requirement can be attributed
to differences in the vitamin C
source. Furthermore, no vitamin C
deficiency signs, such as anorexia,
abnormal swimming, and
hemorrhagic areas under the skin,
could be observed in our study,
in contrast to the study of Ren
et
al.
(2005). Therefore, the dietary
vitamin C requirement of juvenile
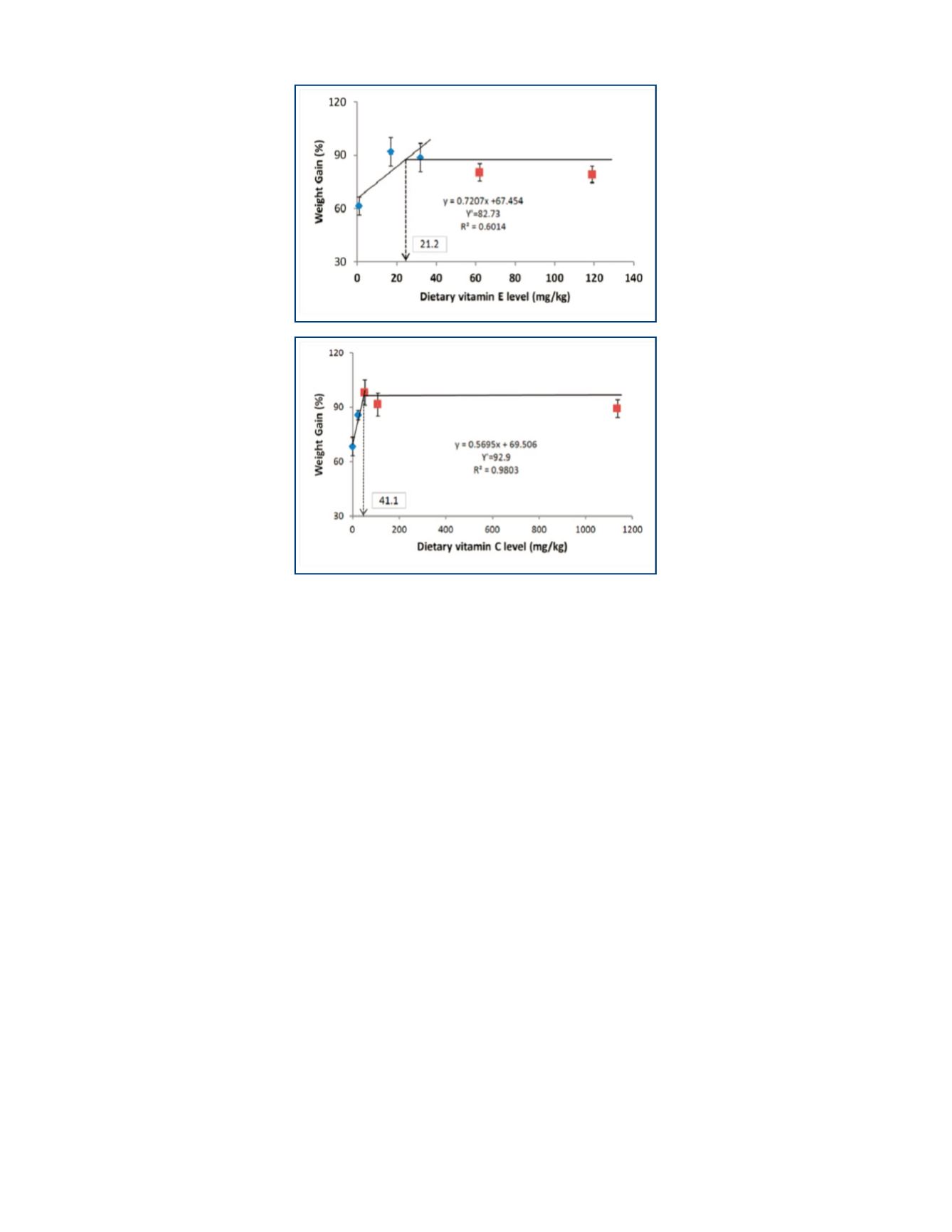
eel is equal to or greater than 41.1
mg/kg diet.
Vitamin E Requirement
Vitamin E (tocopheryl) is a
fat-soluble antioxidant that stops
the production of reactive oxygen
species that are formed when
fat undergoes oxidation. It is an
indispensable nutrient required to
maintain flesh quality, immunity,
normal resistance of red blood
corpuscles to hemolysis, capillary
permeability and heart muscle
(Halver 2002). Vitamin E has
several naturally occurring forms,
with α-tocopherol having the
highest vitamin E activity (NRC
1993). Tocopheryl acetates do not
act as antioxidants but are hydrolyzed by digestive enzymes prior to
absorption into the body (Hung
et al
. 1982, Sau
et al
. 2004).
Vitamin E functions as a lipid-soluble antioxidant, protecting
biological membranes and lipoproteins against oxidation; it is an
essential dietary nutrient for all fish species studied (NRC 1993).
Its main function is to protect unsaturated fatty acids against free
radical-mediated oxidation (Hamre
et al
. 1998). The level and
state of oxidation of polyunsaturated fatty acids in the diet and the
presence of other antioxidants and selenium may affect the dietary
vitamin E requirements of fish (Murai and Andrews 1974, Poston
et al
. 1976, Watanabe
et al
. 1977, Hung
et al
. 1981, Cowey
et al
.
1983, Lovell
et al
. 1984, Gatlin
et al
. 1986). Until recently, there was
no quantitative estimation of the dietary vitamin E requirement for
Japanese eel (Bae
et. al
. 2012).
In our experiment, inclusion of vitamin E did not affect
whole body composition of
Japanese eel. Similar results
were reported by Gatta
et al
.
(2000) and Sau
et al.
(2004),
who found no differences in
lipid, ash or moisture contents
after feeding graded levels
of vitamin E to rohu fry. The
growth performance of fish fed
vitamin E-supplemented diets
improved to a supplementation
level of 16.5 mg TA/kg diet and
then dropped at higher levels.
Based on these observations, the
dietary vitamin E requirement
of the juvenile Japanese eel is
>21.2 but <21.6 mg/kg diet,
as assessed by broken-line
regression analysis of weight gain
(Fig. 4), specific growth rate, feed
efficiency and protein efficiency
ratio. DL-α-tocopheryl acetate
was used as the dietary vitamin
E source under the experimental
conditions in our laboratory.
Arachidonic Acid
Requirement
Among
n
-6 HUFA,
arachidonic acid (ARA, 20:4
n
-6)
is the main fatty acid precursor
of eicosanoids in fish (Henderson
and Sargent 1985, Henderson
et al
.
1985, Bell
et al
. 1994). Arachidonic
acid in fish tissues is located
almost exclusively in the 2-position
of the glycerol of the inositiol
phospholipids, which have critical
roles in many areas of cellular
signal transduction (Sargent
et al
.
1989). Arachidonic acid produces
eicosanoids with high biological
activity, namely 2-series prostanoids and 4-series leukotrienes,
while eicosanoids derived from EPA, namely 3-series prostanoids
and 5-series leukotrienes, are less biologically active (Tocher
et al
.
2003). The relative abundance of the two fatty acids, subsequently,
determines eicosanoid potency and mode of action.
In fishes, eicosanoids are responsible for a range of
physiological functions, such as modulating immune and neural
function and osmoregulation, and controlling the stress response
(Mustafa and Srivastava 1989, Sargent
et al
. 1999, Koven
et al
.
2001b, Tocher
et al
. 2003). Elevated dietary ARA increases overall
survival (Bessonart
et al
. 1999) and improves resistance to handling
stress in larval gilthead seabream
Sparus aurata
(Koven
et al.
2001). An optimal concentration of dietary ARA maximizes stress
resistance to a hypersaline challenge in larval summer flounder
TOP, FIGURE 4.
Broken-line analysis of vitamin E requirement of
Japanese eel based on weight gain.
BOTTOM, FIGURE 5.
Broken-line
analysis of arachidonic acid requirement of Japanese eel based on weight gain.
There are a number of constraints
that need to be resolved to develop a
complete technology package for eel
aquaculture. Poor understanding of
nutrient requirements and the availability
of balanced diets are major barriers to
further expansion of eel aquaculture. The
greatest mortality at eel farms has been
reported during the weaning period and
the adaptation period to dry feed.
( C O N T I N U E D O N P A G E 3 2 )
















