Indonesia: Emerging as a Leader in Aquaculture
EDITOR’S NOTE: As you’ll note on page 6 of this issue, ASIAN PACIFIC AQUACULTURE 2024 will be held i...

Special sessions at the 2020 and 2021 Aquaculture America conferences were developed to highlight the important role aquaculture has come to play in the conservation of threatened and endangered species, and species of concern. The environment for raising aquatic species for commercial production can be significantly different from the conditions needed for rearing conservation species. Therefore, research is needed to evaluate the variable rearing requirements for these species. The researchers herein provide presentations that illustrate how aquaculture tools have been used in conservation to provide refugia, facilitate reproduction and enable supplementation of organisms into the wild.
Ryan Schloesser, Ken Leber, Nathan Brennan and Paula Caldentey
Stock enhancement in estuarine and marine systems of the United States has been limited by the lack of an efficient process to assess the impact of stocking activities. Quantifying stocking success is particularly challenging when recapture rates of released individuals are low or require logistically difficult or extensive effort. As a result, robust monitoring and informed adaptive management remain important issues for stocking programs (Trushenski et al. 2015). The Fisheries Ecology and Enhancement program at Mote Marine Laboratory in Sarasota, FL, has been using the common snook Centropomis undecimalis as a model species for developing an approach to more rapidly estimate post-release mortality. Snook are a popular estuarine sportfish in Florida but are vulnerable to mass mortality from red tide and cold-kill events.

Stock enhancement has been considered as a management option for this species but recapture methodology limits assessment of snook stocking efforts. An alternate approach to assess short-term post-release survival would benefit adaptive management of common snook by informing the most appropriate release locations, times and procedures to support responsible, strategic stock enhancement.
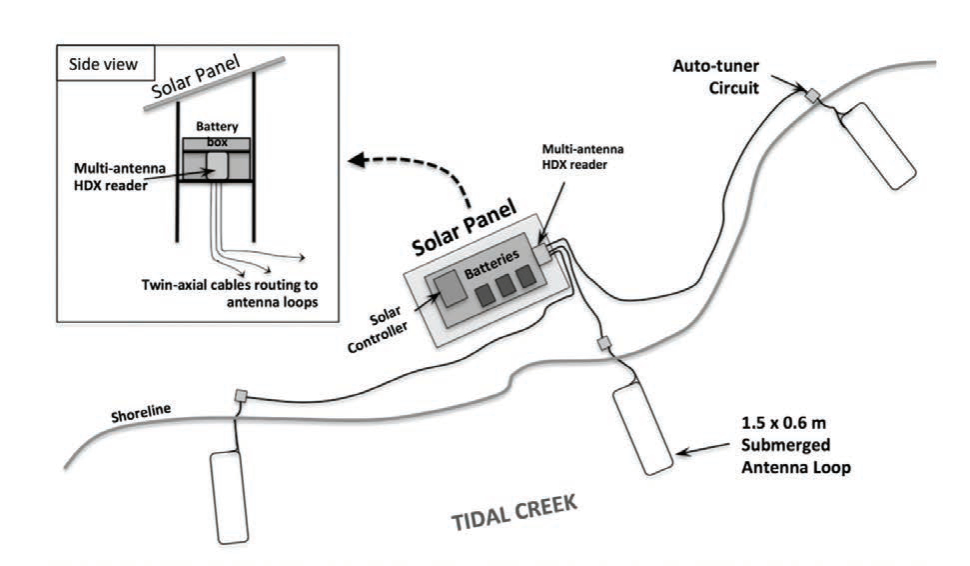
We established an approach that couples the flexibility of release designs for hatchery-reared fishes stocked into estuarine and marine systems with passive integrated transponder (PIT) tag monitoring technology to inform release strategies and improve performance of enhancement efforts (Schloesser et al. in press). The approach was demonstrated in two experiments for which weekly post-release apparent survival was estimated for hatchery-reared fish released into an open, estuarine system. Snook were produced in recirculating aquaculture systems at the Mote Aquaculture Research Park. Broodstock were originally caught from local, wild populations and induced to spawn using photothermal conditioning on two occasions to support a fall and spring release experiments. For each experiment, ~40 individuals were released at each PIT tag antenna array during each of three replicate sub-releases that occurred a week apart (Fig. 1). The antenna array was comprised of three individual 1.5 m × 0.6 m antennas placed along the shoreline to detect fish throughout the water column (Fig. 2). Detections of tagged fish were used to generate resighting histories for the year following each release, and these were analyzed to estimate post-release survival and detectability using multistate open robust design models in program MARK (White et al. 2006), following an approach similar to that of Kendall et al. (2019). State-based models were needed to control for unequal detectability among individuals displaying residency and mobile post-release behaviors.
Monitoring release sites with PIT tag antenna arrays resulted in a high proportion of unique fish resighted in this study (78 percent in fall, 80 percent in spring). For the fall release, 88 percent of the 135,332 detection events occurred within the first four weeks post-release and the number of fish detected decreased substantially during this time as only 64 individuals (6.7 percent) were detected after four weeks.

In the first year after the spring release experiment, the decrease in the number of fish detected over time was not nearly as substantial as it was for the fall release, with 38 percent of the 127,525 detections occurring after four weeks, representing 192 individuals (20 percent of those released).
Resighting histories generated from PIT tag antenna arrays were primarily explained by short-term differences in survival during the first few weeks after release and long-term patterns in detectability that reflect residency behaviors of juvenile snook. For both releases, apparent survival was a function of the interaction between a four- or five-week stocking effect and their post-release behavioral state, the region of release and the size of the fish at release, with time after release also being important for the spring release. Notably, over half of the mortality often occurred within the first week after release and survival of hatchery-reared snook was high beginning five to six weeks post-release. The highest survival rates were observed for individuals released in lower Phillippi Creek (Sarasota) in the spring, suggesting lower reaches of tidal creek systems provide ideal release locations for juvenile snook at this time.
We demonstrated that monitoring stocking activities with PIT tag antenna arrays can be extended to open saline waters, models appropriately account for post-release behavioral states, and the results used to identify times and locations that promote short-term post-release survival without onerous post-release monitoring efforts. Releasing fish in habitats occupied by wild conspecifics does not guarantee effective stocking. Wild juvenile snook occupy tidal creeks over winter but a fall release would have minimal impacts on local snook populations. Specific release site features (e.g., habitat complexity) likely influenced post-release survival but additional environmental conditions need to be examined at a finer spatial scale to further refine release strategies. Juvenile snook may establish residency at small spatial scales, which translates to an increase in persistence and detectability, but was not consistently associated with improved survival. For more mobile or migratory estuarine species, survival could be underestimated if emigration is not considered, making it more important to monitor the full range of the species stocked.
Further application will provide critical insights to guide future stock enhancement strategies, promote adaptive management of stock enhancement programs and maximize the benefits on receiving populations for large-scale stocking efforts. The feasibility of stock enhancement will improve with growing evidence that stocked fish survive and contribute to fisheries and monitoring that supports adaptive management will help ensure stocking of estuarine systems is conducted in a responsible and strategic manner.
Mary L. Moser, Alexa N. Maine, John B. Hume, Kimmo K. Aronsuu, Ralph T. Lampman and Aaron D. Jackson
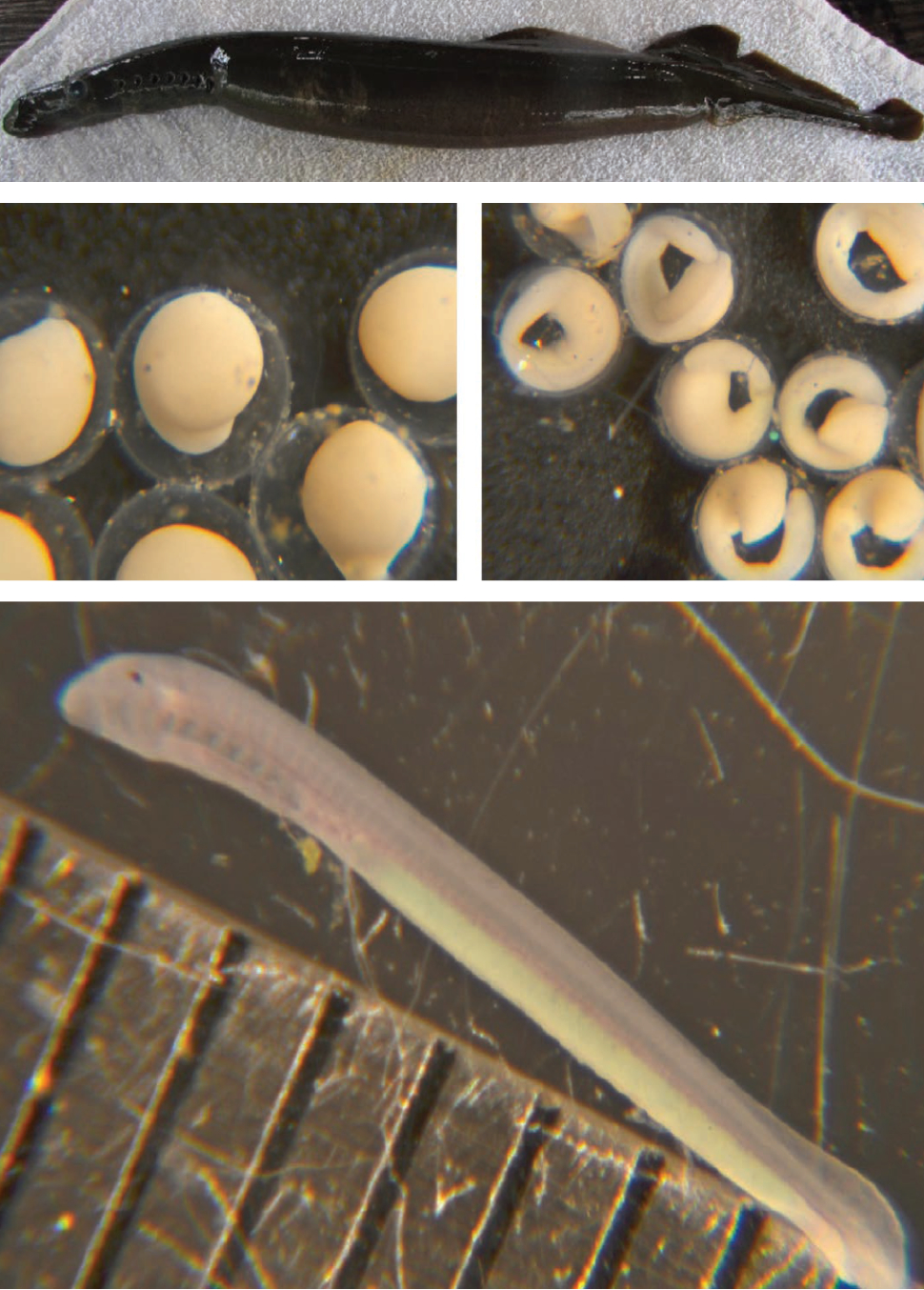
There is a long history of artificial propagation of lampreys (Fig. 3), with recent developments driven by the need for research animals and for recovery of imperiled species. Lamprey culture initially produced specimens for the study of evolutionary development in vertebrates. More recently, artificially propagated larvae have been used to improve identification methods for native lampreys, study invasive sea lamprey Petromyzon marinus in the Laurentian Great Lakes, provide animals for genomic studies and for restoration and conservation.
Broodstock holding has indicated that adult lampreys can be kept at extremely high densities when provided with cold, oxygenated water. Fertilization and incubation experiments revealed that gamete contact times are very short and that embryos are resilient to low flow and poor water quality. Early prolarvae are also resilient to these factors, can tolerate abrupt changes in temperature and extended periods of starvation. However, they cannot survive sudden changes in water quality, excessive disturbance and lack of adequate microbial communities.
These observations have resulted in more efficient and effective lamprey propagation and have yielded important information about the early life stage requirements of lampreys in the wild.
Further study is needed on a broader array of species to allow inter-specific comparisons of early life history. However, information from lampreys receiving the most attention to date (European river lamprey Lampetra fluviatilis, sea lamprey and Pacific lamprey) indicates that culture and environmental requirements of the early life stages are remarkably similar, allowing for generalization across lamprey species.
James Barron, Racheal Headley and Ann Gannam
The Pacific lamprey is an ancient fish of great importance to the ecosystems and indigenous cultures of the U.S. Pacific Northwest. Pacific lamprey have declined in abundance and range from historic levels, thus leading to increased conservation efforts for the species. Efforts to conserve this native fish include development of culture techniques.
The larval stage of this species occurs in freshwater and can take up to seven years to reach metamorphosis in the wild. During the larval stage, lamprey are filter feeders and a slurry of active dry yeast paired with a fine larval fish diet (4:1) is commonly used as a standard diet in the hatchery. Larval lamprey have been successfully cultured with two feeding events per week; however, more frequent feedings could optimize fish growth and condition. This project tested two levels of feeding frequency (two feedings per week, five feedings per week) through two 8-wk trials with 65 days post hatch (DPH) larvae (Trial 1) and 803 DPH larvae (Trial 2).

The high feeding frequency treatment represented a 150 percent increase in feeding events relative to the control. At the end of Trial 1, larvae fed a high frequency were 21 percent heavier on average relative to control fish. Feeding frequency had no significant effect on final length, condition factor or survival.
In Trial 2, high feeding frequency increased length and weight relative to the control fish but did not affect survival or condition factor (Fig. 4). Feeding at a high frequency increased larval weight by 32 percent relative to control fish. High frequency also elevated whole body lipid content by 51 percent relative to the control fish and altered the fatty acid profile. The fatty acid profile of the high frequency fish indicated a build-up of depot lipids in these fish with increased saturates relative to the control fish. Their long chain polyunsaturated fatty acids also decreased relative to the control fish possibly through dilution by the increased depot lipids.
Increasing feeding frequency can increase the growth of the larval lamprey. The effects were more pronounced in older larvae so larger fish may benefit more from increased frequency than young larvae. As the accumulation of sufficient lipid reserves are thought to be critical to reaching metamorphosis in lamprey, the high frequency regime could facilitate a shorter duration of larval rearing. Increased feeding frequency will increase costs and tank fouling so those considerations need to be balanced as a trade-off with increased growth and lipid deposition.
Alexa N. Maine, Mary L. Moser, Aaron D. Jackson and Frank Wilhelm
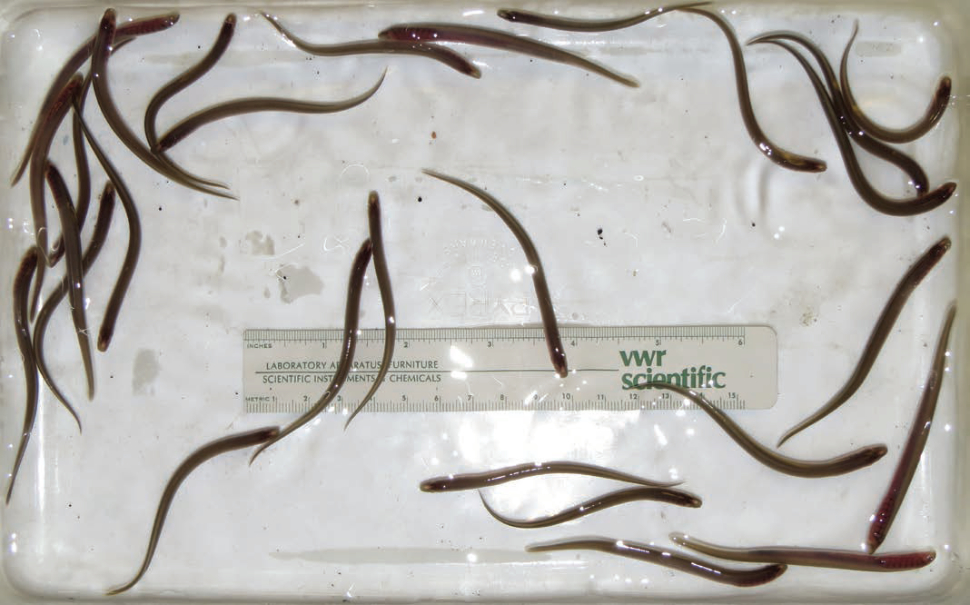
Healthy rivers sustain diverse communities of microorganisms, while aquaculture environments are typically homogenous. Disinfection methods to prevent the development of harmful or beneficial microorganisms can further reduce microbial populations in laboratory cultures. Pacific lamprey Entosphenus tridentatus are reared at Confederated Tribes of the Umatilla Indian Reservation facilities, typically in monoculture (Fig. 5). Because larval lamprey are closely associated with the sediment in their natural environment, they may have an important ecological relationship with the benthic microbial community.
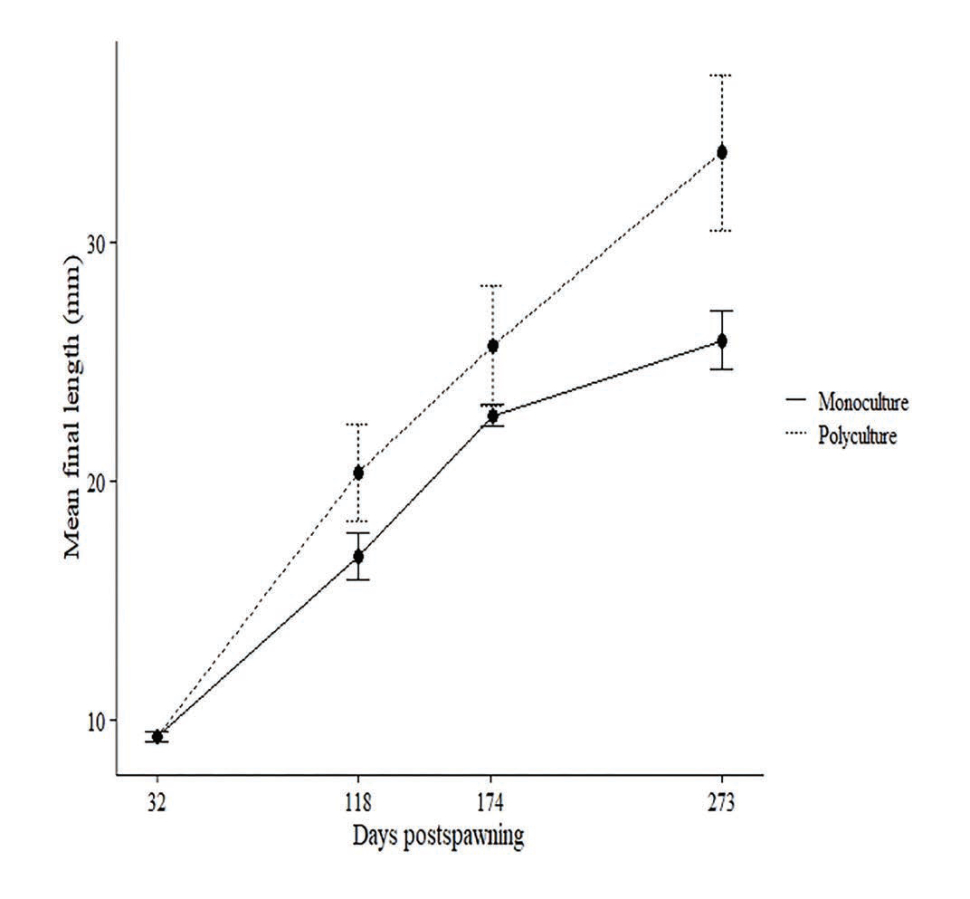
We hypothesized that rearing larval lamprey with other species would improve lamprey growth and survival in a culture setting. We conducted a polyculture experiment in which speckled dace Rhinichthys osculus were held in the same recirculating system with larval lamprey. After 274 days, the size increase of larval lamprey in polyculture (20.5 ± 3.3 mm growth) was significantly greater (p < 0.05) than those in monoculture (16.6 ± 1.3 mm growth; Fig. 6). Survival was not enhanced in polyculture; however, the addition of a filter mat to rearing tanks resulted in increased survival relative to previous cultures without the mat. The filter mat and polyculture with dace likely promoted the development of a more abundant or stable microbial community compared to the monoculture system. Improvements in growth and survival support the idea that microbial interactions are important for these benthic, filter-feeding larvae. This work furthers our understanding of rearing requirements for this culturally important species and highlights the importance of ecological community linkages in aquaculture.
Aaron Pilnick, Keri O’Neil, Matthew DiMaggio and Joshua Patterson
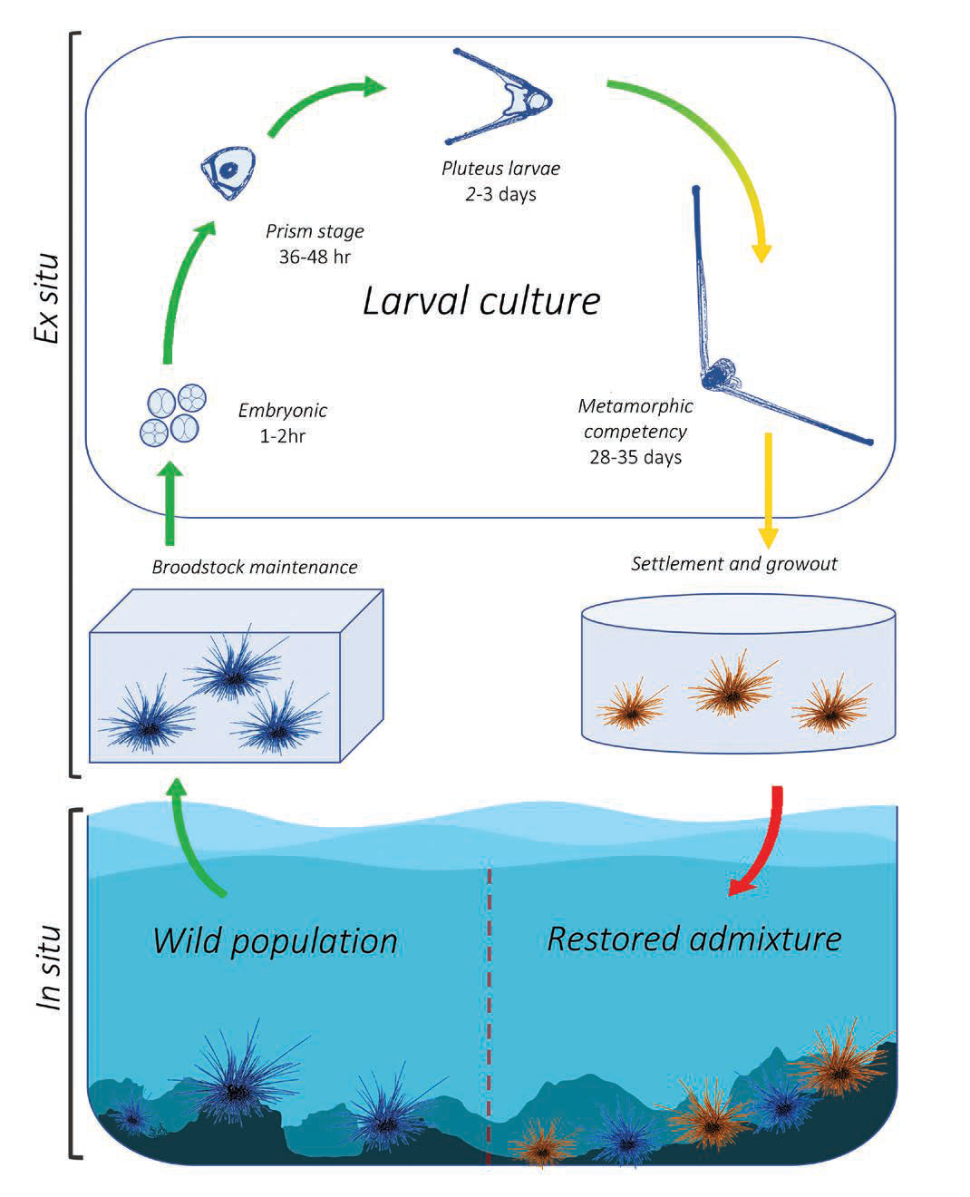
The University of Florida and The Florida Aquarium have been working in partnership to develop aquaculture protocols for the ecologically important long-spined sea urchin Diadema antillarum for coral reef restoration. Once an abundant reef-grazing herbivore throughout the Caribbean, this keystone species is responsible for consuming fleshy macro- and turf algae that competes with reef-building corals (Hughes et al. 2010). In 1983-1984, an unidentified disease caused 93-100 percent mortality of long-spined sea urchin populations throughout its native range, resulting in a sudden lack of herbivory and contributing to an ecological phase shift from hard coral to macroalgae-dominated reef ecosystems (Lessios 2016). This species has not recovered substantially and the loss of functional herbivory has strongly contributed to ongoing Caribbean coral reef declines (Miller et al. 2003). Reef restoration practitioners are therefore greatly interested in augmenting reef herbivory by restoring populations to pre-mortality densities. These objectives would benefit from scalable methods to sexually propagate this species from gametes and restock to the wild (Fig. 7). Development of long-spined sea urchin aquaculture has realized limited success over the past ~20 years due to an extremely difficult and lengthy larval culture process of around 40 days and lack of existing information. Beginning in 2018, researchers based at the Florida Aquarium Center for Conservation (CFC) in Apollo Beach have been working to address these knowledge gaps.
Although commercial echinoderm aquaculture methods are well established, aspects of long-spined sea urchin larval biology are unique and prevent application of these general methods for successful development in culture. Primarily, the negatively buoyant and mechanically fragile echinopluteus larvae necessitated the creation of a unique semi-circular culture vessel with pulsed aeration (Leber et al. 2008, Moe 2014). Long-spined sea urchin also exhibit extreme sensitivity to water quality, specifically dissolved heavy metals (Bielmyer et al. 2005), and likely have specific nutritional requirements (Eckert 1998). A novel experimental recirculating system was designed and built at the CFC to investigate appropriate larval diet requirements within a hatchery production setting (Pilnick et al. 2021).
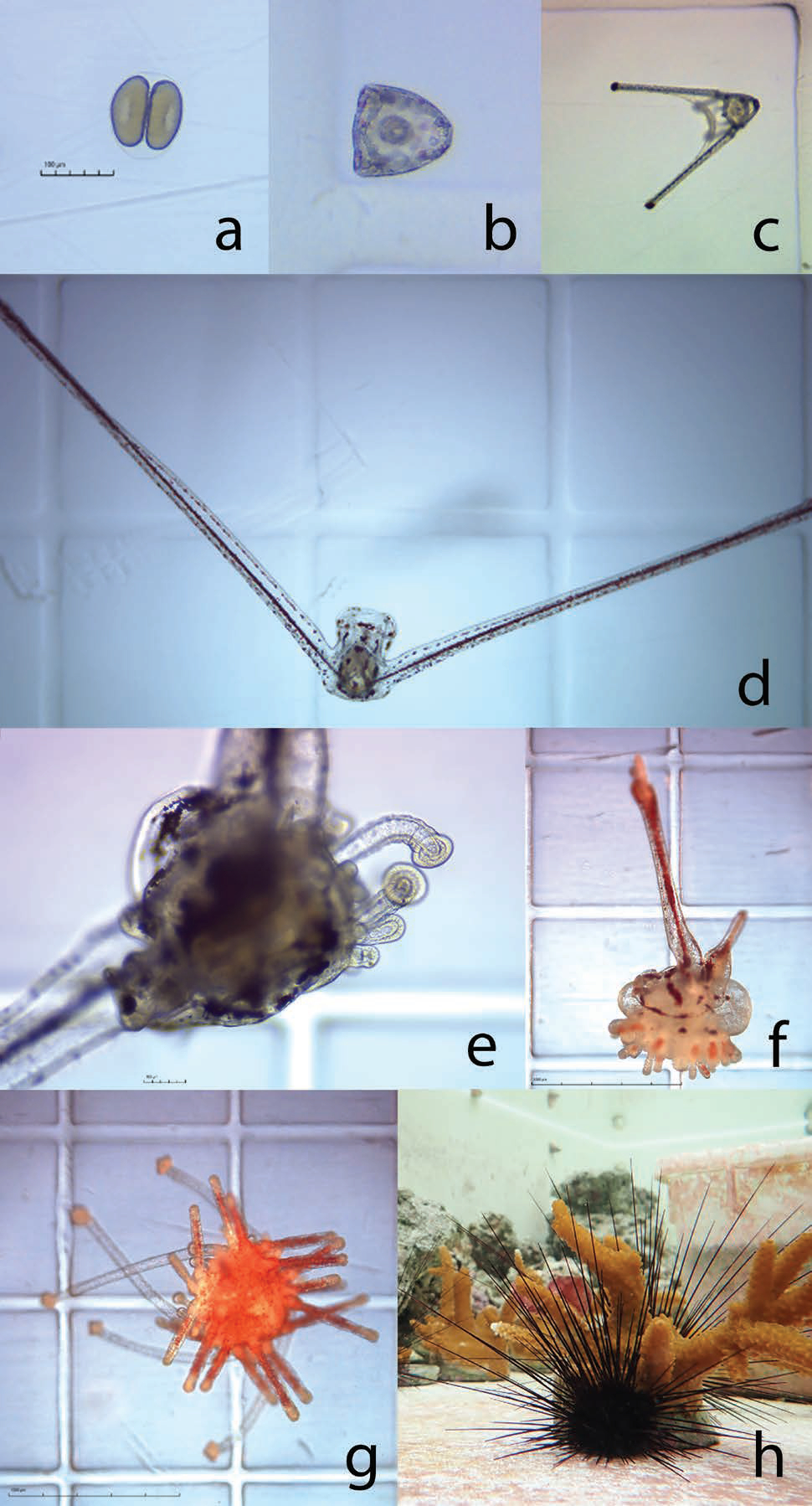
An initial trial established a reference diet containing two microalgae species, Tisochrysis lutea and Chaetoceros sp., and compared larval success between a high density (40,000 cells/mL) diet and a low density (10,000 cells/mL) diet over 21 days-post fertilization. The average larval growth rate per day was 6.6 percent in the high-density treatment, however larval body size and survival was similar overall between treatments. Further experimentation comparing the effects of different carbon-equivalent microalgae diet combinations revealed enhanced performance from specific diets. In one experiment, two diets containing Rhodomonas lens resulted in significantly larger larvae and a higher proportion of surviving larvae compared to the reference diet after 21-days post-fertilization.
Data generated were used to establish fundamental larviculture protocols that have since produced over 700 juvenile long-spined sea urchin (Fig. 8). Although these efforts represent the most successful instances of long-spined sea urchin aquaculture to date, more research is needed before being able to fully implement a successful hatchery production process for restoration.
Renee E. Angwin, Brian T. Hentschel and Todd W. Anderson
Sea urchins are an edible delicacy worldwide. Also known as uni or roe, urchin gonads are a highly prized seafood in Europe and Asia, especially in Japan. Only 17 of the over 850 species of urchins are commercially valuable, and only two of those – red urchin Mesocentrotus franciscanus and purple urchin Strongylocentrotus purpuratus – are native to the west coast of the United States (Harris and Eddy 2015). The red sea urchin fishery began in 1971 in southern California to develop an underutilized species and to curb destructive grazing on giant kelp (Kalvass and Rogers-Bennet 2002).
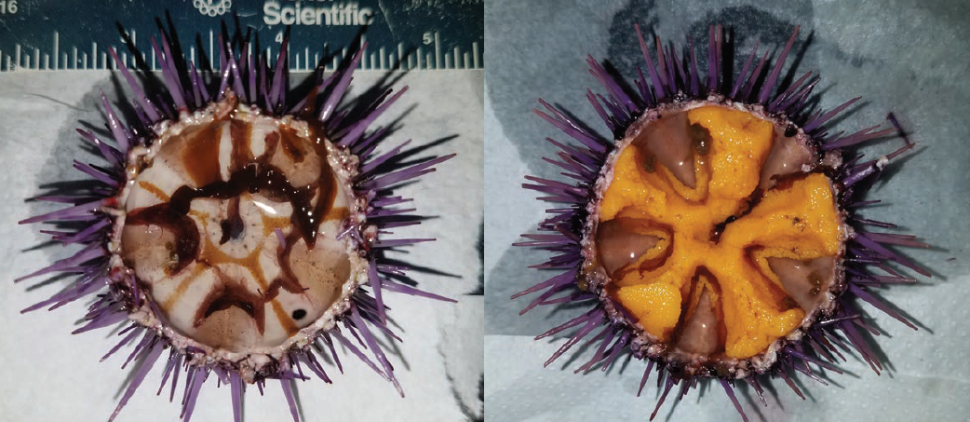
In contrast, purple sea urchins have very limited commercial value due to their smaller size and lower roe yield, and a robust fishery for purple urchins has not yet fully developed (Parker and Ebert 2002). Purple urchins have long been considered a pest because they voraciously consume kelp (Parker and Ebert 2002, House et al. 2017). In recent years, overgrazing by purple urchins has transformed lush kelp forests into barren grounds devoid of kelp and associated inhabitants (Ling et al. 2015, Filbee-Dexter and Scheibling 2014). This formation of urchin barrens has been largely attributed to the loss of top predators and increasing oceanic temperatures, both of which create opportunistic windows for urchins to overgraze kelp habitat as natural controls and sources of kelp replenishment are altered (Ling et al. 2015, Filbee-Dexter and Scheibling 2014). Along the California coast, high densities of purple urchins have resulted in more than 93 percent loss of kelp in some locations (CDFW Aerial Kelp Surveys 2016). These herbivores subsist on very little food and can rapidly acclimate to adverse conditions, making them challenging to control (Kelly et al. 2013, Ling et al. 2015). Catastrophic regime shifts to urchin barrens are difficult to reverse; virtually all urchins must be removed to facilitate the return of kelp (Ling et al. 2015), which can only occur through severe disturbance (Ebeling et al. 1985). Malnourished purple urchins within barrens (Fig. 9) have little to no roe and presently have no economic value.
With global catches of commercially harvested urchin species in decline, interest in urchin aquaculture, also known as echinoculture, has been increasing (Liyana-Pathirana et al. 2002, Dale et al. 2005, Cuesta-Gomez and Sanchez-Saavedra 2017, 2018, Baiao et al. 2019). Removing purple urchins from barrens and ranching them for roe enhancement can create an economically viable product that also contributes to kelp forest restoration. Using land-based aquaculture techniques in conjunction with specially formulated prepared feeds, barren urchins can be transformed from empty, malnourished pests into roe-rich seafood in less than ten weeks (Gagnon et al. 2017, James et al. 2017). Roe enhancement is more economically sustainable than traditional echinoculture that begins with larvae that require up to three years to grow to adult size (Eddy et al. 2015, Walker et al. 2015), and trials have shown promising results with Evechinus chloroticus in New Zealand (James 2006), Strongylocentrotus droebachiensis in Norway and Canada (James et al. 2017, Gagnon et al. 2017), and Loxechinus albus in Chile (Lawrence et al. 1997). Roe enhancement is not currently employed in the United States and has not been tested rigorously on S. purpuratus.
The recent expansion of barrens caused by overabundant purple urchins provides an enormous opportunity to sustainably exploit these high-density populations and transform their low-quality gonads into a valued seafood resource. The outcome of such an opportunity can help reinvigorate the California urchin industry, restore kelp forests from degraded barrens and enhance the ecosystem services kelp forests provide to impacted coastal communities. In this study, we collected purple urchins from barrens and fed them whole kelp or two different formulations of algal-based prepared feed in a recirculating aquaculture system (RAS). The gonad index (GI) of urchins fed the two prepared diets doubled in 6 weeks, increased from a barren condition to a marketable yield (GI > 15 percent) in nine weeks (Fig. 9), and was significantly greater than the GI of urchins fed kelp. Analyses of proximate constituents and amino acid composition of gonad tissue also revealed differences among the roe of urchins fed the three diets for 9 weeks. In particular, one of the two prepared feeds resulted in a significantly greater amount of bitter-tasting amino acids in enhanced gonads compared to urchins fed kelp, while the other prepared formulation indicated a more natural flavor profile, suggesting superior suitability for commercial applications. Our results highlight an untapped potential to produce quickly a highly valued seafood product from seemingly low-value purple urchins. Echinoculture could thereby serve as a tool to stimulate urchin industries that also facilitates the restoration of kelp forests from urchin barrens.
Luke Gardner, Helaina Lindsey, Katherine Neylan, Walan Chang, Katrina Herrmann, Max Rintoul and Katherine Roy
Sea urchin barrens are characterized by an overabundance of sea urchins and coralline algae where kelp forests once dominated a marine ecosystem. The prevalence and duration of these barrens appears to be increasing globally on temperate coastlines. This is of concern for marine resource managers as the barrens generally result in a reduction in habitat diversity and productivity. Recently, California and Oregon, USA, are undergoing an ecosystem switch from kelp forests to urchin barrens, which are persisting and expanding from the Central California coast north into Oregon. Significant reduction of urchin populations from barrens usually results in restoration of kelp forests. However, the large geographical scale of this barren represents a logistical challenge for economic urchin removal. Conventional fisheries mediated removal of urchins has not been possible due to the urchins typically possessing minor gonad development necessary for commercial marketing of gonads as uni. In response to this, aquaculture combined with fisheries have been used to enhance the urchin gonad quality and thereby the value of these wild urchins to incentivize their capture.

Although sea urchin roe enhancement is not a novel concept, technical limitations to the activity are primarily related to the availability of macroalgae diets given seasonality and the propensity of urchin barrens to deplete kelp forests. Development of sustainable alternative diets for urchins is necessary for future commercial urchin aquaculture. A 2019 study aimed to enhance roe from purple sea urchins Strongylocentrotus purpuratus collected from California urchin barrens using four diet treatments, including giant kelp Macrocystis pyrifera, ogo Gracilaria pacifica, formulated commercial diet (Urchinomics), and an unfed control. During the 10-wk study, gonadal somatic index (GSI) was measured in a subset of five urchins from each replicate tank every two weeks. Baseline GSI at the beginning of the trial was < 0.5 percent. A commercially marketable GSI of 10 percent was reached most rapidly in the formulated diet treatment at 6 weeks, followed by ogo and kelp at 9 and 13 weeks respectively (Fig. 10). The relative short duration to culture purple urchins to a marketable gonad condition is promising, especially considering the variation in time to GSI score among the different diets. The variation in diet response indicates that there is significant scope for further husbandry and nutritional improvement.
This preliminary examination of the feasibility of urchin ranching in California indicated the biological potential to develop urchin gonads using alternative diets with a view to restore kelp forests and develop a nascent aquaculture industry in California. Future research for roe enhancement of purple urchins should include a more thorough assessment of nutritional requirements and digestibility, as well as quantitatively assessing the resulting roe for important market characteristics including color, texture and taste. An economic feasibility is also required to determine the commercial potential of this aquaculture activity for restoring kelp forests. The potential of commercial aquaculture to be used to achieve a conservation goal and economic profitability is an uncommon opportunity and should be thoroughly investigated.
Justin Crow
Although aquaculture has become an increasingly important tool for meeting the needs of a growing human population in a sustainable manner, it also plays a critical role in the conservation of at-risk species. Captive assurance and breeding colonies of amphibians have been established by universities, non-profits, zoological institutions and conservation agencies around the world. The International Union for Conservation of Nature and Natural Resources listed captive programs among the top response priorities relevant to amphibian conservation globally.


The San Marcos Aquatic Resources Center, a US Fish and Wildlife Service facility, is an aquaculture facility dedicated to the support of federally listed species (Fig. 11). The mission of the Center is to provide support for and undertake research on endangered, threatened and species at risk. The Center maintains captive assurance colonies for multiple amphibian species, including the Texas blind salamander Eurycea rathbuni, San Marcos salamander Eurycea nana, Barton Springs salamander Eurycea sosorum (Fig. 12), and the Houston toad Anaxyrus (Bufo) houstonensis. Aquaculture-related activities for these species are inherent to this mission.
William Haines, Katrina Scheiner, Kelli Konicek, Karl Magnacca and Cynthia King
The damselfly genus Megalagrion is endemic to Hawaiʻi and includes about 25 species, many of which have suffered major declines due to habitat loss and the introduction of mosquito fish Gambusia spp. that prey on the aquatic immature stages (naiads) of damselflies. The once common orangeblack Hawaiian damselfly Megalagrion xanthomelas (Fig. 13) was thought to be extinct on Oʻahu until it was rediscovered in 1994 at Tripler Army Medical Center (TAMC). This small population occupies only about 100 m of a narrow streambed in which water flow is maintained by a running faucet. The population is therefore highly vulnerable and establishment of additional populations on the island is a top priority.
In November 2018, the Hawaii Department of Land and Natural Resources (DLNR) began a captive rearing and reintroduction program for M. xanthomelas in which eggs were collected from TAMC, hatched and reared in a laboratory, then released as last-stage immatures or adults into fish-free habitats. Large numbers of naiads could be successfully raised in the laboratory with low mortality. By 2020, DLNR had released over 1,000 captive-reared naiads at two locations: artificial ponds at Lyon Arboretum in the Koʻolau Range on East Oʻahu and a natural stream in the Waiʻanae Range on West Oʻahu. Unfortunately, neither reintroduction was successful, but for different reasons. At Lyon Arboretum, adult damselflies successfully emerged and mated, but were not attracted to the fish-free ponds that had been provided to them. Instead, they favored nearby taro patches that were heavily infested with mosquito fish., The site was considered nonviable because it was not feasible to remove fish from the taro patches. At the stream in the Waiʻanae Range, preliminary results were more promising. Damselflies were observed laying eggs in the fish-free, spring-fed stream. However, a second generation of damselflies was never observed at the site, possibly because it is located near the high elevation limit for this species and winter stream water temperatures dropped below the range of temperatures known to be suitable for the species at other sites. Since 2020, DLNR has continued to rear M. xanthomelas in captivity, and is now focusing on a third reintroduction site in northwest Oʻahu. Damselflies were released from June 2020 to June 2021. Monitoring of the site is ongoing, but damselflies have successfully produced a second generation, and a small population persists. DLNR is working with their US Army collaborators to enhance habitat at the site with the aim of increasing its carrying capacity.
Kelli Konicek, William Haines, Katrina Scheiner and Cynthia King
Captive rearing of M. xanthomelas naiads produced large numbers of naiads with a high survival rate, but naiads reared in the laboratory were much lighter than those observed in the wild. The reasons for this were unclear and DLNR staff were concerned that the pale coloration may increase naiads’ vulnerability to predation when they were released into the wild, or that it reflected a nutrient or mineral deficiency in the naiad’s diet or environment in the laboratory.

Rearing methods were adjusted to test the effects of diet supplementation and substrate type on naiad developmental rates and pigmentation. To measure the effect of diet diversity, naids were fed a diet consisting of exclusively brine shrimp Artemia franciscana or a diet supplemented with a diversity of freshwater zooplankton cultured from the wild. Naiads fed a more diverse diet developed significantly faster but were only slightly darker than naiads fed only brine shrimp.
To explore the roles of substrate color and composition, naiads were reared on six different substrates spanning a range of colors and textures. Substrate color had a much greater effect on naiad coloration, with naiads raised on dark backgrounds more closely matching the color of wild naiads than those raised on lighter backgrounds (Fig. 14). Based on these results DLNR has modified rearing protocols for this endangered insect. Naiads are now fed zooplankton and insect larvae to promote faster growth, and rearing cups are lined with dark gravel to produce a more camouflaged pigmentation.

Because there was little previous work with mass rearing of damselflies, and no previous work on husbandry of the genus Megalagrion, the development of rearing protocols has yielded much valuable information on the life history of this rare insect. Now that researchers are able to produce rapidly thousands of healthy naiads, doors have been opened for conservation of Hawaiian damselflies. Now the limiting factor is not whether large numbers of individuals can be reared, but whether suitable reintroduction sites can be found or created.
Ann Gannam, Abernathy Fish Technology Center, U.S. Fish and Wildlife Service, 1440 Abernathy Creek Rd., Longview, WA 98632
Ryan Schloesser, Mote Marine Laboratory, Sarasota, FL 34236 rschloesser@mote.org
Mary L. Moser, Northwest Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric
Administration, 2725 Montlake Boulevard East, Seattle, Washington, 98112. mary.moser@noaa.gov
Alexa N. Maine, Confederated Tribes of the Umatilla Indian Reservation, Pacific Lamprey Project, Pendleton, OR, University of Idaho, Environmental Science Program, Moscow, ID. AlexaMaine@ctuir.org
James Barron, Abernathy Fish Technology Center, U.S. Fish and Wildlife Service, 1440 Abernathy Creek Rd, Longview, WA 98632. jmbarron@bpa.gov
Aaron Pilnick, School of Natural Resources and Environment, University of Florida | IFAS 103 Black Hall, Gainesville, FL 32611. apilnick@ufl.edu
Luke Gardner, California Sea Grant, Scripps Institution of Oceanography, 9500 Gilman Drive, Dept. 0232, La Jolla, CA 92093, Moss Landing Marine Laboratories, 8272 Moss Landing Rd., Moss Landing, CA 95039. lgardner@ucsd.edu
Renee E. Angwin, SDSU Coastal and Marine Institute Laboratory, 4165 Spruance Rd, Suite 100 San Diego, CA 92101. rangwin@sdsu.edu
Justin Crow, San Marcos Aquatic Resources Center, U.S. Fish & Wildlife Service, San Marcos, TX 78666. justin_crow@fws.gov
William Haines, Hawaii Invertebrate Program, Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife,University of Hawaii, Center for Conservation Research and Training, 3050 Maile Way, Gilmore 310, Honolulu, HI 96822. whaines@hawaii.edu
Kelli Konicek, Hawaii Invertebrate Program, Hawaii Department of Land and Natural Resources, Division of Forestry and Wildlife, University of Hawaii, Center for Conservation Research and Training, 3050 Maile Way, Gilmore 310, Honolulu, HI 96822. kkonicek@hawaii.edu The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the U.S. Fish and Wildlife Service.
Baiao, L.F., F. Rocha, M. Costa, T. Sa, A. Oliveira, M.R.G. Maia, A.J.M. Fonseca, M. Pintado and L.M.P. Valente. 2019. Effects of protein and lipid levels in diets for adult sea urchins Paracentrotus lividus (Lamarck, 1816). Aquaculture 506:127-138.
Bielmyer, G.K., K. v. Brix, T.R. Capo and M. Grosell. 2005. The effects of metals on embryo-larval and adult life stages of the sea urchin, Diadema antillarum. Aquatic Toxicology 74:254-263.
California Department of Fish and Wildlife. 2016. Aerial Kelp Survey. www.wildlife.ca.gov/Conservation/Marine/Kelp/Aerial-Kelp-Surveys (accessed 6 August 2019).
Cantu, V., J.C. Crow and K. Ostrand. 2016. A comparison of two non-invasive spawning methods to genetically manage captive Barton Springs salamanders Eurycea sosorum. Herpetological Review 47:59-63.
Cuesta-Gomez, D.M. and M.P. Sanchez-Saavedra. 2017. Effects of protein and carbohydrate levels on survival, consumption and gonad index in adult sea urchin Strongylocentrotus purpuratus (Stimpson 1857) form Baja California, Mexico. Aquaculture Research 48:1596-1607.
Cuesta-Gomez, D.M. and M.P. Sanchez-Saavedra. 2018. Effects of dietary protein and carbohydrate levels on gonad index, composition, and color in the purple sea urchin Strongylocentrotus purpuratus. North American Journal of Aquaculture 80:193-205.
Dale, T., S.I. Siikavupopio and K. Aas. 2005. Roe enhancement in sea urchins: effects of handling during harvest on transport mortality and gonad growth in Strongylocentrotus droebachiensis. Journal of Shellfish Research 24:1235-1239.
Ebeling, A.W., D.R. Laur and R.J. Rowley. 1985. Severe storm disturbances and reversal of community structure in a southern California kelp forest. Marine Biology 84:287-294.
Eddy, S.D., N.P. Brown and L.G. Harris. 2015. Aquaculture of the green sea urchin Strongylocentrotus droebachiensis in North America. Pages 175-209 in N.P. Brown and S.D. Eddy, eds. Echinoderm Aquaculture. John Wiley & Sons, Inc., Hoboken, New Jersey.
Filbee-Dexter, K. and R.E. Scheibling. 2014. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Marine Ecology Progress Series 495:1-25.
Fries, J.N. 2002. San Marcos salamander upwelling flow velocity preferences of captive adult San Marcos salamanders. North American Journal of Aquaculture 64:113-116.
Gagnon, P., D. Belanger and S. Trueman. 2017. Investigation of a novel approach to land-based production of green sea urchin roe in Newfoundland. Project report to Green Seafoods Ltd.
Harris, L.G. and S.D. Eddy. 2015. Sea urchin ecology and biology. Pages 3-24 in N.P. Brown and S.D. Eddy, eds. Echinoderm Aquaculture. John Wiley & Sons, Inc., Hoboken, New Jersey.
House, P., A. Barilotti, H. Burdick, T. Ford, J. Williams, C. Williams and D. Pondella. 2017. Palos Verdes Kelp Forest Restoration Project. Project Year 4 July 2016-June 2017: Report to the Bay Foundation. 46 pp.
Hughes, T.P., N.A.J. Graham, J.B.C. Jackson, P.J. Mumby and R.S. Steneck. 2010. Rising to the challenge of sustaining coral reef resilience. Trends in Ecology and Evolution 25:633-642.
James, P.J. 2006. A comparison of roe enhancement of the sea urchin Evechinus chloroticus in sea-based and land-based cages. Aquaculture 253:290-300.
James, P., T. Evansen and A. Samuelsen. 2017. Commercial scale sea urchin roe enhancement in Norway: Enhancement, transport and market assessment. Nofima Report No. 7/2017.
Kalvass, P. and L. Rogers-Bennett. 2002. Red sea urchin. Pages 9-1 to 9-14 in C. Ryan and M. Patyten, eds. Annual Status of the Fisheries Report through 2003. California Department of Fish and Wildlife, Belmont, CA. Web. nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=34394&inline.
Kelly, M.W., J. Padilla-Gamino and G. Hofman. 2013. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Global Change Biology 19:2536-2546.
Kendall W.L., S. Stapleton, G.C. White, J.I. Richardson, K.N. Pearson and P. Mason. 2019. A multistate open robust design: population dynamics, reproductive effort, and phenology of sea turtles from tagging data. Ecological Monograms 89:e01329. doi.org/10.1002/ecm.1329
Leber, K., K. Lorenzen, K. Main, M. Moe, D. Vaughan, T. Capo, A. Bardales and P. Gilette. 2008. Developing restoration methods to aid in recovery of a key herbivore, Diadema antillarum, on Florida coral reefs. Protect Our Reefs Grant - 2008 Progress Report. Mote Technical Report #1295.
Lessios, H.A. 2016. The great Diadema antillarum die-off: 30 years later. Annual Review of Marine Science 8:267-283.
Ling, S.D., R.E. Scheibling, A. Rassweiler, C.R. Johnson, N. Shears, S.D. Connell, A.K. Salomon, K.M. Norderhaug, A. Perez-Matus, J.C. Hernandez, S. Clemente, L.K. Blamey, B. Hereu, E. Ballesteros, E. Sala, J. Garrabou, E. Cebrian, M. Zabala, D. Fujita and L.E. Johnson. 2015. Global regime shift dynamics of catastrophic sea urchin overgrazing. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 370:20130269.
Liyana-Pathirana, C., F. Shahidi and A. Whittick. 2002. The effect of an artificial diet on the biochemical composition of the gonads of the sea urchin (Strongylocentrotus droebachiensis). Food Chemistry 79:461-472.
Moe, M. 2014. Breeding the West Indian sea egg: Tripneustes ventricosus. CORAL Magazine 11:80-94.
Miller, R.J., A.J. Adams, N.B. Ogden, J.C. Ogden and J.P. Ebersole. 2003. Diadema antillarum 17 years after mass mortality: Is recovery beginning on St. Croix? Coral Reefs 22:181-187.
Parker, D. and T. Ebert. 2003. Chapter 10. Purple Sea Urchin. Pages 10-1 to 10-4, in C. Ryan and M. Patyten, eds. Annual Status of the Fisheries Report through 2003. California Department of Fish and Wildlife, Belmont, CA. Web. https://nrm.dfg.ca.gov/FileHandler.ashx?DocumentID=34393&inline (accessed 22 Sept 2017).
Pilnick, A.R., K.L. O’Neil, M. Moe and J.T. Patterson. 2021. A novel system for intensive Diadema antillarum propagation as a step towards population enhancement. Scientific Reports 11:1-13.
Schloesser R.W., K.M. Leber, N.P. Brennan and P. Caldentey. in press. A novel approach to estimate post release survival for estuarine fishes. Bulletin of Marine Science 97(4). doi.org/10.5343/bms.2020.0040
Trushenski, J.T., H.L. Blankenship, J.D. Bowker, T.A. Flagg, J.A. Hesse, K.M. Leber, D.D. MacKinlay, D.J. Maynard, C.M. Moffitt and V.A. Mudrak. 2015. Introduction to a special section: hatcheries and management of aquatic resources (HaMAR) –considerations for use of hatcheries and hatchery-origin fish. North American Journal of Aquaculture 77:327-342.
Walker, C.W., S.A. Boettger, T. Unuma, S.A. Watts, L.G. Harris, A.L. Lawrence and S.D. Eddy. 2015. Enhancing the commercial quality of edible sea urchin gonads - technologies emphasizing nutritive phagocytes. Pages 263-286 in N.P. Brown and S.D. Eddy, eds. Echinoderm Aquaculture. John Wiley & Sons, Inc., Hoboken, New Jersey.
White, G.C., W.L. Kendall and R.J. Barker. 2006. Multistate survival models and their extensions in program MARK. Journal of Wildlife Management. 70:1521-1529.