IDENTIFICATION, MOLECULAR CHARACTERISATION OF INTERLEUKIN-1ß (IL-1 ß) HOMOLOG FROM YELLOWTAIL CLOWNFISH Amphiprion clarkii
Interleukin -1 β or IL-1 β is a cytokine that is a member of the β-trefoil cytokine family. It is produced as inactive proprotein and activated through cleavage by various enzymes. The mature peptide exerts its effect by binding with type 1 IL-1 receptors and thereby initiating the signaling. The IL-1 β gene is basically considered as a pro-inflammatory cytokine and produced by many cell types.
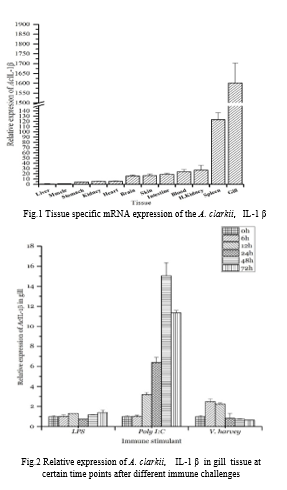
IL-1 β sequence was identified from the constructed cDNA library of Clark’s anemonefish. Bioinformatics software and online tools were employed to characterize the identified nucleotide sequence and predicted the amino acid sequence. Healthy fish were dissected and 12 tissues including peripheral blood leucocytes, head kidney, kidney, liver, gill, and spleen were harvested to isolate mRNA and synthesize cDNA. The cDNA was used to evaluate the transcriptional difference of IL-1 β in different tissues in A. clarkii. Healthy fish were challenged with IP injections of LPS, Poly I:C and live Vibrio harveyi. Gills were dissected at 0, 6, 12, 24, 48 and 72 hour time points after the challenge. mRNA isolation and cDNA synthesis were performed and the qPCR was used to investigate the fold change of IL-1 β mRNA transcription.
A. clarkii, IL-1 β gene is composed of 741 bp ORF, 83bp 5’ UTR and 397bp 3’ UTR. The predicted amino acid sequence composed of 246AA. Predicted MW is 28.1kDa. Phylogenetic analysis show cladding with known fish counterparts. The multiple sequence analysis shows the IL-1 β sequence has conserved IL-1B superfamily domain within known fish IL-1 β sequences. In mRNA expression analysis shows the highest expression in gills and spleen. In the immune challenge experiment, IL-1 β was more responsive to Poly I:C treatment. However, the poly I:C treatment shows late phase induction of IL-1 β this might due to the activation of secondary immune stimulants. The IL-1 β was immediately upregulated in live Vibrio harveyi challenge but with low intensity compared to Poly I:C. These results suggest that IL-1 β playing an important role against viral and bacterial pathogens in A. clarkii.