ISOLATION AND IN VITRO CULTURE OF PRIMARY GERM CELLS DERIVED FROM OVARIAN TISSUES OF WHITE AND BLACK CRAPPIE
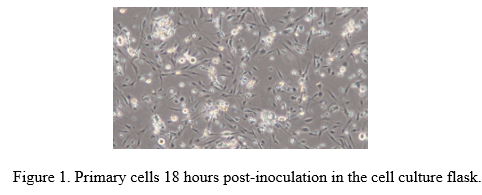
Investigations were conducted to develop a general protocol for the isolation and in vitro culture of ovary-derived germ cells in Black crappie (Pomoxis nigromaculatus) and White crappie (Pomoxis annularis). Ovarian tissues were obtained from one-year-old Black and White crappie. Five different digestive enzymes: 0.25% trypsin-EDTA, 0.05% trypsin-EDTA, 500U/mL collagenase type I, 500U/mL collagenase type IV, and TrypLETM Express were evaluated for cell isolation. Cells isolated from ovarian tissues were cultured in two different concentrations (10%, 20%) of Fetal Bovine Serum (FBS) in the L-15 growth media. In addition, four incubation temperatures (15, 20, 25, and 30°C) were also evaluated to determine optimal culture conditions for these ovarian germ cells. Viable cells were obtained from all enzyme treatments. However, the number of viable cells obtained from the 0.25% trypsin and TrypLETM Express treatments were significantly higher than the other treatments. No significant effects were observed in cell growth between the 10% and 20% FBS treatments. Cells isolated using TrypLETM Express and 0.25% trypsin attained 80-90% confluency in 25 mm2 cell culture flasks within five days of inoculation in 20, 25, and 30°C incubation regimes. At a 15°C incubation temperature, the isolated cells took ten days post-inoculation to reach 80-90% confluency. Upon microscopic inspection, cells incubated at 20 and 25°C appeared morphologically healthier than cells incubated at 30°C, where cell detachment from the substrate and irregular cell shape was observed. Based on these findings, we concluded that TrypLETM Express and 0.25% trypsin were optimal for cell disassociation and isolation, while an incubation temperature of 20-25°C was conducive for cell culture in L-15 media supplemented with either 10 or 20% FBS.