IDENTIFICATION OF RECEPTORS OF PIRABVP BINARY TOXIN RELEASED BY Vibrio parahaemolyticus CAUSING ACUTE HEPATOPANCREATIC NECROSIS DISEASE (AHPND) IN Penaeus vannamei SHRIMP
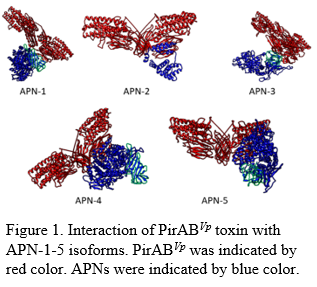
Figure 1. Interaction of PirABVp toxin with APN-1-5 isoforms. PirABVp was indicated by red color. APNs were indicated by blue color.
Acute hepatopancreatic necrosis disease (AHPND) in shrimp is caused by Vibrio parahaemolyticus , an enteric pathogen carrying a large plasmid which encodes a binary toxin, PirABVp . The tertiary structure of PirABVp toxin shows similarity to CryBt toxin produced by Bacillus thuringiensis that causes lethal infection in insects. In insect, aminopeptidase N (APN) is one of the receptors of CryBt toxin, but the receptors in shrimp foregut and hepatopancreas that bind to PirABVp remain unknown. Upon mining NCBI database, five isoforms of insect homologs of APN in shrimp (Penaeus vannamei ) were identified. Similar to insect APNs, shrimp APNs contain a transmembrane domain, a Cry Binding Region (CBR) and two signature amino acid sequence motifs. Phylogenetic analyses showed that crustacean APNs are similar to insect APNs but form a separate clade. In silico analysis of the tertiary structure of PirABVp and predicted tertiary structure of shrimp APNs revealed all APN isoforms displayed the binding affinity to PirABVp. Interestingly, following an experimental challenge of Specific Pathogen Free (SPF) P. vannamei shrimp with AHPND-causing V. parahaemolyticus , none of the five APN transcripts were detected in the foregut, and four out of five APN isoforms, APN-1, -2, -4 and -5 were detected by qRT-PCR in the hepatopancreas tissue.
The mRNA expression of APN-4 and -5 were significantly higher in an AHPND susceptible than in a tolerant shrimp line. Modelling of proposed biologically functional PirABVp toxin and APN interactions coupled with the mRNA expression data suggest while APNs serve as receptors of binary toxin in hepatopancreas, and in the gut PirABVp toxin likely binds to other receptors. In addition, lower mRNA expression of APN-4 and -5 in hepatopancreas of AHPND-tolerant line suggests a reduction in toxin uptake by epithelial cells in hepatopancreatic tubule may contribute to AHPND resistance. Collectively, these findings shed new insights on the molecular mechanisms of PirABVp toxin involved in V. parahaemolyticus pathogenesis in shrimp.